
MACROPHAGE PHAGOCYTOSIS AND MOTILITY
Dr. Dianne Cox, Ph.D.
Professor, Albert Einstein College of Medicine
E-mail: dianne.cox@einstein.yu.edu
 |
Dr. Dianne Cox, Ph.D.
|  |
Macrophage Phagocytosis and Motility |
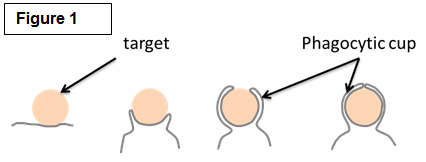
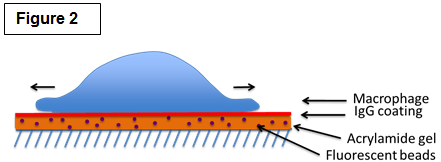
| Studying the molecular mechanisms of phagocytosis Among their many roles, macrophages are best known for their striking ability to engulf a large number of big (>0.5µm) particles that are very diverse in size and shape in a process called phagocytosis. Phagocytosis is important in many situations such as the clearance of pathogen and particles (bacteria, yeast, pollen and pollutants). Crucially, phagocytosis may also be a major player of cancer immunity by mediating the engulfment and killing of cancer cells. Phagocytosis requires actin assembly, pseudopod extension, and phagosome closure (Figure 1). Actin polymerization in response to particle binding requires the activation of members of the Rho GTPases, either Rac or Cdc42 for Fc gamma Receptor-mediated phagocytosis or Rho in the case of CR3-mediated phagocytosis. Both Rac and Cdc42 regulate the cytoskeleton in part through the activation of the Wiskott Aldrich Syndrome/ WASP verprolin-homologous (WASP/WAVE) family of proteins. RhoG, another family member, is also important for phagocytosis but the precise role of RhoG is currently unknown. We are exploring the roles of these signaling pathways as well as those regulating the myosin family of molecular motors in phagocytosis. We are employing a novel technique called traction force microscopy (Figure 2) to understand the roles of these factors in the protrusive forces needed to engulf the diverse particles of various sizes and stiffnesses found in nature. |
 |
 |
| Several human pathogens, such as Cryptococcus neoformans and Legionella pneumophila, are internalized by macrophages, escape destruction and grow and divide inside the macrophage. The phagocytic processes by which these organisms are internalized are currently unknown. We are dissecting the downstream signaling pathways that mediate these processes during phagocytosis. |
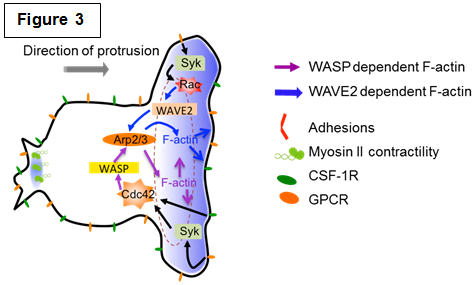
| Studying the molecular mechanisms of chemotaxis The directed movement of cells in response to chemoattractants involves several complex, interrelated processes, including directional or chemotactic sensing, polarity, and motility. These processes are mediated by complex, interacting signaling pathways that appear to have many similarities but yet have distinct characteristics depending on the chemoattractant and receptor. Many of the signaling cascades utilized for phagocytosis are also required for chemotaxis yet they result in the appearance of different structures (Figure 3 and our review [Rougerie et al., Immunol. Rev. 2013]). We are currently dissecting the signaling pathways required for macrophage chemotaxis towards: 1. CSF-1, a growth factor for macrophage survival and differentiation produced by many tumors and found in high concentrations in arthritic joints; 2. Chemokines that direct monocyte recruitment to different tissues. |
 |
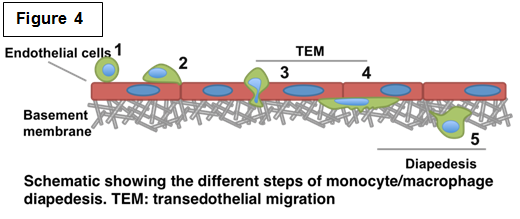 Determining the role of podosomes during monocyte/macrophage diapedesis Determining the role of podosomes during monocyte/macrophage diapedesis Recruitment of immune cells from the blood to sites of tissue damage and infection is crucial. However, prolonged inflammation could have deleterious effects leading to serious chronic inflammatory diseases such as ischemic reperfusion and rheumatoid arthritis. Therefore understanding the mechanisms by which monocytes/macrophages contribute to this process would have a significant impact on identifying pharmacological targets and strategies. Post injury inflammation features the release of pro-inflammatory mediators that activate circulating monocytes as well as the vascular endothelium. We are interested in deciphering the mechanism of this multistep monocyte-endothelium interaction known as diapedesis (Figure 4). This involves the ability of cells to adhere to vascular endothelium, migrate across the endothelial barrier followed by degradation of sub-endothelial matrix. Monocyte-derived cells form characteristic F-actin rich structures known as podosomes, which are known to be required for cell adhesion, motility and degradation through multiple barriers. We are currently investigating the different regulators of podosomes and how they regulate the different roles that podosomes could potentially play during diapedesis. As key regulators of the actin cytoskeleton, our lab and others have shown that Cdc42 and downstream effectors WASP and N-WASP are essential regulators of podosome formation and/or function. Using different 2D and 3D in vitro models, we are currently investigating the role of podosomes during the different steps of diapedesis. In addition, using live imaging we are interested in monitoring the dynamics of podosomes during this process. Interestingly, using our newly developed FRET-based biosensor for Cdc42 (Hanna and Miskolci et al. PLoS One 2014), we will be able to monitor the function of Cdc42 during diapedesis in both space and time. |
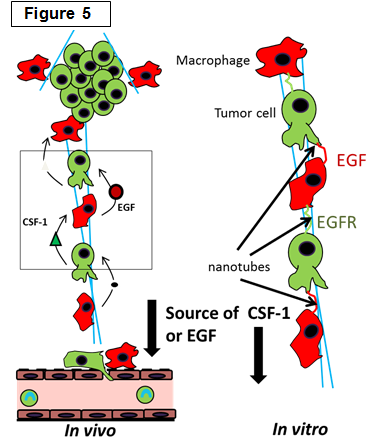
Determining the role of macrophages in the tumor microenvironment |
 |
| Development of tools for live cell imaging In order to determine the role of individual molecules in macrophage functions it is essential to understanding the timing and localization of specific protein involved. We have been actively involved in the generation of new probes for single live cell imaging including photoconvertible actin probes that label various structures in live cells (Figure 6A,). We have also developed and employed biosensors to monitor localized protein activity in live cells (Figure 6B, Cammer et al., J. Biol. Chem. 2009), including the recent work in collaboration with Dr. Louis Hodgson on the development of isoform specific single chain RhoGTPase biosensors (Hanna and Miskolci et al., PloS One 2014). |
Figure 6: (A) Continuous actin polymerization is required for podosome lifetime. Dendra-actin is present in existing podosomes (green) and, following photoconversion (0), red cytoplasmic actin is added to the podosome. (B) Active WASP is present in chemoattractant induced protrusions in macrophages. |
Selected Publications (Students in bold)
Abou-Kheir, W., Gevrey, J-C., Issac, B.M., Yamaguchi, H., and Cox, D. (2005) A WAVE2/Abi1 complex mediates CSF-1-induced F-actin rich membrane protrusions and migration in macrophages. J. Cell Science 118:5369-5379.
Abou-Kheir, W.G., Isaac, B., Yamaguchi H., and Cox, D. (2008) Membrane targeting of WAVE2 is not sufficient for WAVE2 dependent actin polymerization: a role for IRSp53 in mediating the interaction between Rac and WAVE2. J. Cell Science 121:379-90.
Cammer, M., Gevrey, J-C., Lorenz, M., Dovas, A., Condeelis, J. and Cox, D. (2009) The mechanism of CSF-1 induced WASp activation in vivo: A role of PI 3-kinase and Cdc42. J. Biol. Chem. 284:23302-11.
Luo, Y., Isaac, B.M., Casadevall, A., and Cox D. (2009) Macrophage phagocytosis suppresses F-actin enriched membrane protrusions stimulated by CX3CL1 and CSF-1. Infect. and Immun. 77:4487-4495.
Isaac, B.M.*, Ishihara, D.*, Nusblat, L.M., Gevrey, J-C, Dovas A., Condeelis, J., and Cox, D. (2010) N-WASP has the Ability to Compensate for the Loss of WASP in Macrophage Podosome Formation and Function. Exp. Cell Res. 316:3406-16. *co-first authors
Dovas, A., Gligorijevic, B., Chen, X., Entenberg, D., Condeelis, J. and Cox, D. (2011) Visualisation of actin dynamics in invasive structures of macrophages and carcinoma cells using photoconvertible b-actin – Dendra2 fusion proteins. PLoS One 6:e16485.
Nusblat, L.M., Dovas, A., and Cox, D. (2011) The essential role of N-WASP in podosome-mediated matrix degradation. Eur. J. Cell Biol. 90:205-212.
Ishihara, D., Dovas A., Park, H., Isaac, B.M., and Cox, D. (2012) The chemotactic defect in Wiskott-Aldrich Syndrome macrophages is due to reduced persistence of directional protrusions. PLoS One 7(1):e30033 PMCID:PMC3261183
Dovas. A., Patsialou, A., Harney, A.S., Condeelis, J. and Cox, D. (2012) Imaging interactions between macrophages and tumor cells that are involved in metastasis in vivo and in vitro. J. Microscopy doi: 10.1111/j.1365-2818.2012.03667
Ishihara, D.*, Dovas, A.*, Hernandez, L., Pozzuto, M., Wyckoff, J., Segall, J., Condeelis, J., Bresnick, B., and Cox, D. (2013) Wiskott-Aldrich syndrome protein regulates leukocyte-dependent breast cancer metastasis. Cell Reports 4:429-436 *co-first authors
Rougerie, P., Miskolci, V. and Cox, D. (2013) Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunol. Reviews. 256:222-239.
Park, H., Dovas, A., Hanna, S., Cougoule, C, Marridoneau-Parini, I., and Cox, D. (2014) Tyrosine phosphorylation of Wiskott-Aldrich Syndrome protein (WASP) by Hck regulates Macrophage Function. J. Biol. Chem. 289(11):7897-906.
Hanna, S.*, Miskolci, V.*, Cox, D.# and Hodgson, L.# (2014) Development of a new single-chain genetically encoded Cdc42 FRET biosensor. PLoS One 9(5):e96469. *co-first authors, #co-corresponding authors
Miskolci, V., Hodgson, L., Cox, D., and Vancurova, I. (2014) Western Analysis of Intracellular Interleukin-8 in Human Mononuclear Leukocytes. Methods in Molecular Biology: Cytokines. 1172:285-93.
Miskolci, V., Spiering, D., Cox, D., and Hodgson, L. (2014) A mix-and-measure assay for determining the activation status of endogenous Cdc42 in cell lysates. Methods in Molecular Biology: Cytokines. 1172:173-84.
Wu, B.*, Miskolci, V.*, Donnelly, S.K., Cox, D., Singer, R.H.# and Hodgson L.# (2015) Synonymous modification of repeated sequences in retroviral reporters. Genes Dev. In press. *co-first authors, #co-corresponding authors